Microscopy plays an important role in research and development of new drug formulations, but is also essential for the quality control of pharmaceutical products. Since the size range of pharmaceutical particles and emulsions reaches the lower end of the nanometre scale, classical light microscopy is increasingly replaced by electron microscopy techniques.
Pharmaceuticals
How can we help you?
• Quality Control
• Micro- and Nanostructure of Liquids, Emulsions, Lipids, Liposomes and Micelles
• Qualitative and Quantitative Identification of Filter Residues
• Distribution of Active Ingredients and Filling Materials in Tablets
• Correlative Large Area Analyses of Solids (in the Range of cm² with SEM/EDX, FTIR and Raman)
Your Contact Details

DI Dr. Johannes Rattenberger
Key-Account-Manager – Pharma
Tel. +43 316 873-8339
Contact me

Ing. Hartmuth Schröttner
Gruppenleiter REM/IR/Raman
Tel. +43 316 873-8349
Contact me
Morphology and structure
Pharmaceutical developers and manufacturers need the high resolution of the SEM to characterise, control and analyse the size and shape of unwanted particulates in pharmaceutical products. Since pharmaceutical systems are often sensitive to electron irradiation, special techniques like low-voltage SEM, environmental SEM (ESEM) and cryo-methods like freeze-fracture are used.
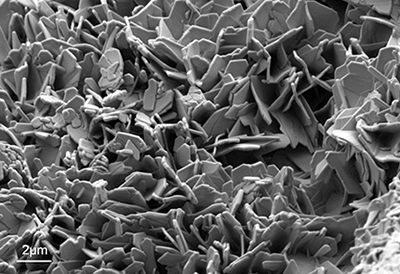
SEM-image of a crystalline drug powder recorded at low electron energy.
Pharmaceutical powder analysis
(example Mg powder)
The combination of the depth of focus and material contrast of the SEM (BSE) with the depth resolution and chemical sensitivity of the confocal Raman microscope make this analysis of particles in a powder without any sample preparation possible.
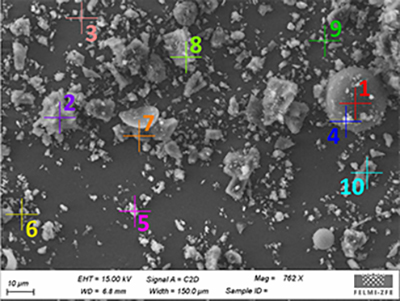
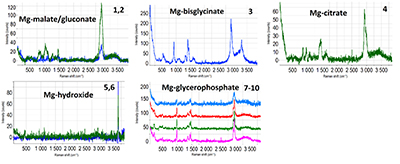
Chemical imaging of pharmaceutical
phases
Pharmaceutical tablets are composed of different materials each contributing to the therapeutic effect of the active ingredient. It is important to analyse not only the distribution of active ingredients, but also of the excipients (fillers, bulking agents, coatings). Therefore, quality control mechanisms mostly rely on the imaging of chemical phases by FT-IR spectroscopy and Raman microspectroscopy.
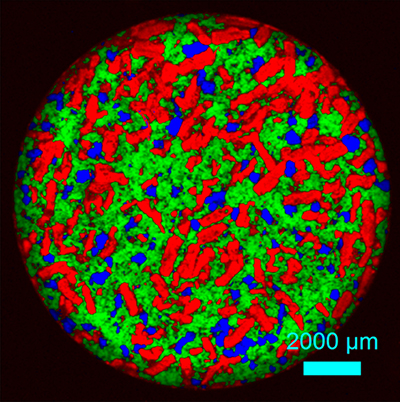
Raman-image of a pharmaceutical tablet showing the distribution of the chemical phases; acetylsalicylic acid (red), paracetamol (green) and coffein (blue).
Cryo-TEM of nanoemulsions
Cryo-TEM enables the investigation of aqueous samples (lipid nanocarriers, nanoemulsions), where the cellular structure and composition are maintained under physiological conditions. By rapid cooling to liquid nitrogen temperature, molecular movements are stabilised and the structure is preserved and observed in a cryo-TEM in its natural state.
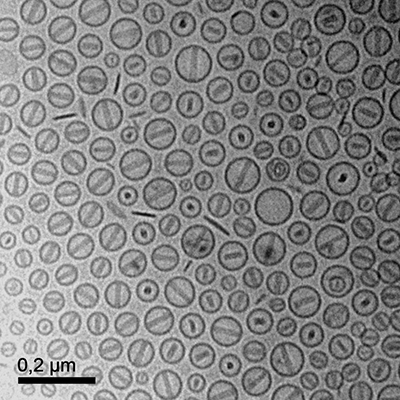
Cryo-TEM image of liposome particles in vitreous ice.